
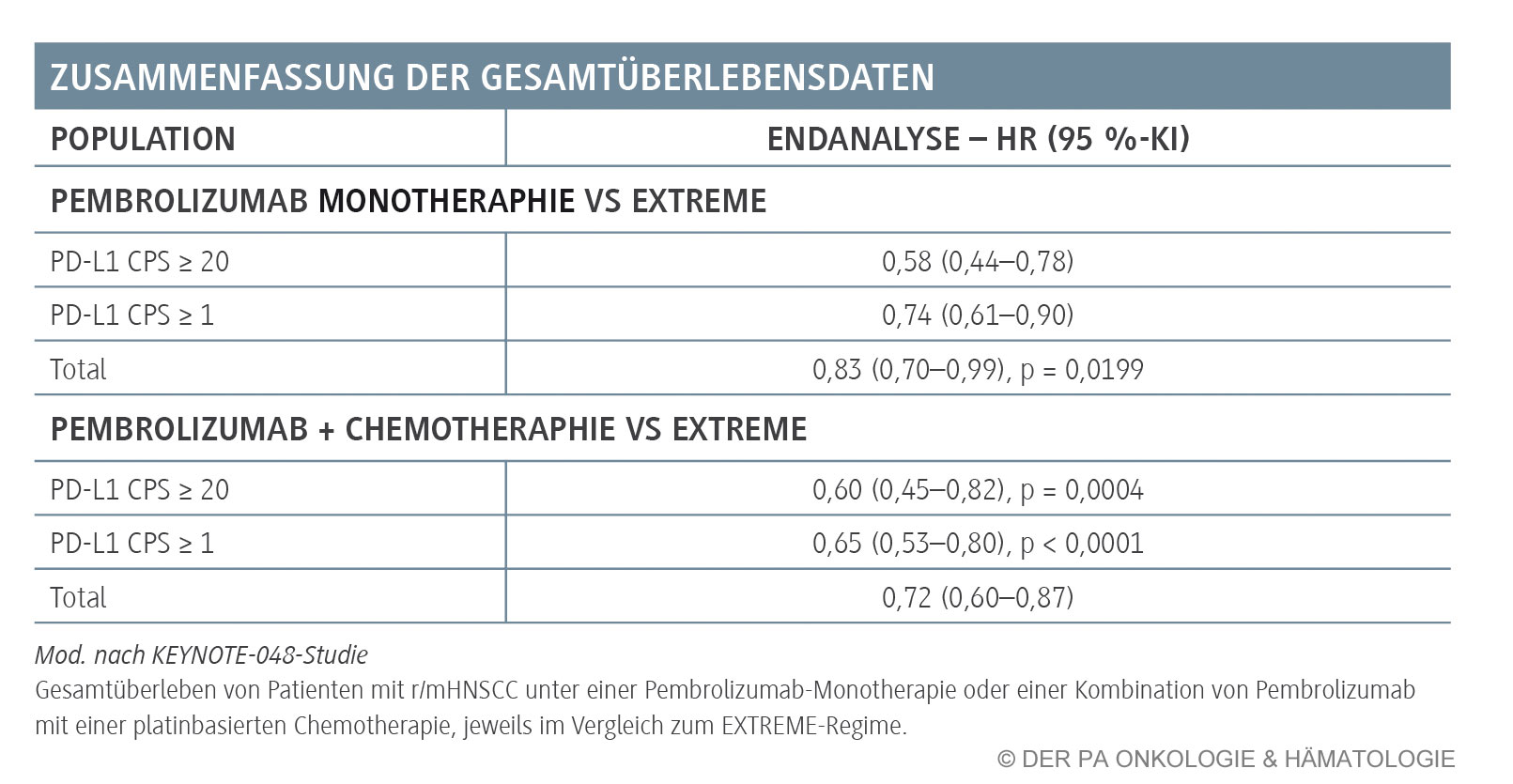
Interpretation Based on the observed efficacy and safety, pembrolizumab plus platinum and 5-fluorouracil is an appropriate first-line treatment for recurrent or metastatic HNSCC and pembrolizumab monotherapy is an appropriate first-line treatment for PD-L1-positive recurrent or metastatic HNSCC.Results: 882 pts were randomized: 301 to P, 281 to P + C, 300 to E. Adverse events led to death in 25 (8%) participants in the pembrolizumab alone group, 32 (12%) in the pembrolizumab with chemotherapy group, and 28 (10%) in the cetuximab with chemotherapy group. At final analysis, grade 3 or worse all-cause adverse events occurred in 164 (55%) of 300 treated participants in the pembrolizumab alone group, 235 (85%) of 276 in the pembrolizumab with chemotherapy group, and 239 (83%) of 287 in the cetuximab with chemotherapy group. Neither pembrolizumab alone nor pembrolizumab with chemotherapy improved progression-free survival at the second interim analysis.
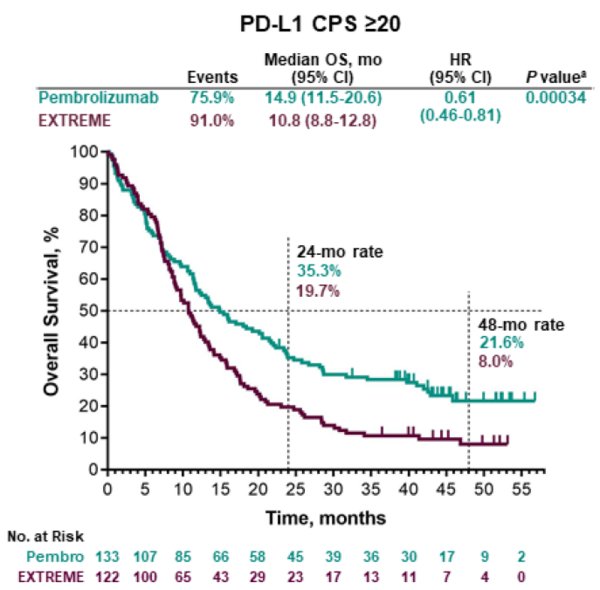
Pembrolizumab with chemotherapy improved overall survival versus cetuximab with chemotherapy in the total population (13.0 months vs 10.7 months, HR 0.77, p=0.0034) at the second interim analysis and in the CPS of 20 or more population (14.7 vs 11.0, 0.60, p=0.0004) and CPS of 1 or more population (13.6 vs 10.4, 0.65, p<0.0001) at final analysis. At the second interim analysis, pembrolizumab alone improved overall survival versus cetuximab with chemotherapy in the CPS of 20 or more population (median 14.9 months vs 10.7 months, hazard ratio 0.61, p=0.0007) and CPS of 1 or more population (12.3 vs 10.3, 0.78, p=0.0086) and was non-inferior in the total population (11.6 vs 10.7, 0.85 ). Findings Between April 20, 2015, and Jan 17, 2017, 882 participants were allocated to receive pembrolizumab alone (n=301), pembrolizumab with chemotherapy (n=281), or cetuximab with chemotherapy (n=300) of these, 754 (85%) had CPS of 1 or more and 381 (43%) had CPS of 20 or more. This study is registered at, number NCT02358031. Safety was assessed in the as-treated population (all participants who received at least one dose of allocated treatment). The definitive findings for each hypothesis were obtained when statistical testing was completed for that hypothesis this occurred at the second interim analysis for 11 hypotheses and at final analysis for three hypotheses. There were 14 primary hypotheses: superiority of pembrolizumab alone and of pembrolizumab with chemotherapy versus cetuximab with chemotherapy for overall survival and progressionfree survival in the PD-L1 CPS of 20 or more, CPS of 1 or more, and total populations and non-inferiority (noninferiority margin: 1.2) of pembrolizumab alone and pembrolizumab with chemotherapy versus cetuximab with chemotherapy for overall survival in the total population.
KEYNOTE 048 FREE
The primary endpoints were overall survival (time from randomisation to death from any cause) and progression- free survival (time from randomisation to radiographically confirmed disease progression or death from any cause, whichever came first) in the intention-to-treat population (all participants randomly allocated to a treatment group). Investigators, participants, and representatives of the sponsor were masked to the PD-L1 combined positive score (CPS) results PD-L1 positivity was not required for study entry. Investigators and participants were aware of treatment assignment. Participants were stratified by PD-L1 expression, p16 status, and performance status and randomly allocated (1:1:1) to pembrolizumab alone, pembrolizumab plus a platinum and 5-fluorouracil (pembrolizumab with chemotherapy), or cetuximab plus a platinum and 5- fluorouracil (cetuximab with chemotherapy). Methods KEYNOTE-048 was a randomised, phase 3 study of participants with untreated locally incurable recurrent or metastatic HNSCC done at 200 sites in 37 countries. LANCET, v.394, n.10212, p.1915-1928, 2019īackground Pembrolizumab is active in head and neck squamous cell carcinoma (HNSCC), with programmed cell death ligand 1 (PD-L1) expression associated with improved response. MESIA, Ricard NGAMPHAIBOON, Nuttapong RORDORF, Tamara ISHAK, Wan Zamaniah Wan HONG, Ruey-Long MENDOZA, Rene Gonzalez ROY, Ananya ZHANG, Yayan GUMUSCU, Burak CHENG, Jonathan D. GREIL, Richard SOULIERES, Denis TAHARA, Makoto CASTRO JR., Gilberto de PSYRRI, Amanda BASTE, Neus NEUPANE, Prakash BRATLAND, Ase FUEREDER, Thorsten HUGHES, Brett G. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 studyīURTNESS, Barbara HARRINGTON, Kevin J. Please use this identifier to cite or link to this item:


 0 kommentar(er)
0 kommentar(er)
